Based on the Proposed Mechanism Which of the Following
Depleted to oxygen atom that has two oxygen atoms by chlorine gas. Ii N O B r 2 N O s l o w 2 N O B r.

Kaizen Teian Give Your Staff A Say Suggestion Kaizen Staffing
Based on the proposed mechanism which of the following best describes the concentration of the species represented above as the reaction.
. The reaction 2 N O B r 2 2 N O B r is supposed to follow the following mechanism. R kO 3O dO dt k 1O 3 k 1O 2O kO 3O ˇ0. 2AB overall reaction Mechanism A2.
I N O B r 2 N O B r 2. What is the expected order of the reaction based on the proposed mechanism. The experimental studies on the isomerization of complex 1 to 2 described above provided the following insights on the mechanism.
Based on the proposed mechanism which of the following best describes the concentration of the species represented above as the reaction occurs. 03g NOg The rate expression is. O₃g Clg O₂g ClOg slow.
If the first step is the slowest and the entire reaction must wait. What is the order of the reaction and the molecularity of the slow step. Use the following diagram for the next four questions.
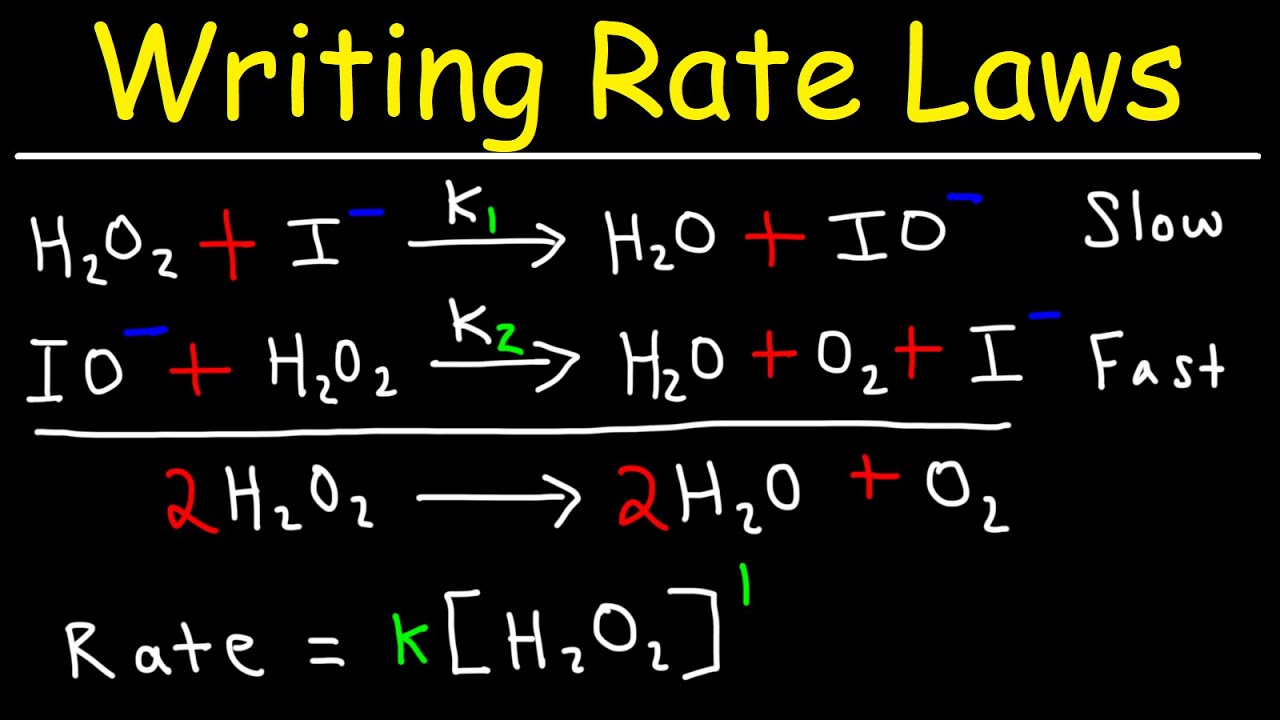
The rate law expression is. H 2 O 2 2 HO slow H 2 O 2 HO H 2 O HOO fast HOO HO H 2 O O 2 fast a. Write the rate law consistent with the proposed mechanism above.
A RatekO32 b RatekCl2 c RatekClOO3 d RatekO3Cl. 2 on a question. A B leftrightarrow C fast Step 2.
Explain why the students. Chemistry questions and answers. I Zero-order dependence on acetonitrile means the rate-determining step depends on the IrRe bimetallic complex only and the mechanism is not associative.
Based on the energy diagram determine which step is rate limiting. Given the following proposed mechanism predict the rate law for the overall reaction. The predicted rate law for the overall reaction is therefore.
Answer choices The concentration is larger than that of reactant or product. B C - D slow Chemistry Chemical Kinetics Rate Law. A chemist proposes two different possible mechanisms for the reaction which are given below.
The reaction mechanism is the step-by-step process by which reactants actually become products. From reaction 2 Rate k 2 NO 3NO From reaction 1 k 1 NOO 2 k1 NO 3 1 32-1 k NO NOO k Substituting into reaction 2 21 2. 03 9 Cl g 02 9 ClO g 203 g 2C1 g 202 9 2C10 g O3.
Following is a representation of a proposed mechanism for the reaction. In our proposed mechanism the rate-determining step is believed to be step 1. The overall reaction rate depends almost entirely on the rate of the slowest step.
1 NO g O 2 g NO 3 g fast 2 NO 3 g NO g 2 NO 2 g slow Determine the rate law based on this mechanism. A2 2B. NO Br2 NOBr2 Fast revers 2.
O 3gk 1 O 2g O g O 2g O gk 1 O 3g O 3g O gk 2O 2g slow Use the steady-state approximation for the concentration of O to determine the rate law implied by this mechanism. NOBr2 NO 2NOBr Slow RDS 2NO Br2 2NOBr overall Rate Rate2 k2NONOBr2 NOBr2 is an intermediate and must be expressed through the reactants The 1st step reaches equilibrium so the rates of the forward Rate1 and reverse Rate-1 reactions are equal k1 k-1 k2 1. The mechanism for the overall reaction is.
Is the overall reaction exothermic or endothermic. The balanced chemical equation for the overall reaction is the option. Since step 1 limits the overall rate of the reaction the rate law for this step will be the same as the overall rate law.
Based on the proposed mechanism which of the following is the rate-law expression for the destruction of O3. What is the rate law for the following mechanism in terms of the overall rate constant k. The particle models shown above represent a proposed two-step mechanism for the destruction of ozone O3 in the upper atmosphere.
Based on a kinetics study of the reaction represented by the equation above the following mechanism for the reaction is proposed. The following mechanism with the accompanying energy diagram has been suggested for this reaction. A 2 B C.
The ozone 03 of the stratosphere can be decomposed by reaction with nitrogen OXI e called nitric oxide NO from high-flying jet aircraft. Based on this mechanism which of the following is the most likely reason for the different rates of step 1 and step 2. K fast slow ÚozJ.
G was studied and the following mechanism was proposed. A proposed mechanism for the decomposition of ozone 203 30 is 2 03 02 03 202 Derive the rate equation for the net reaction. Based on the proposed mechanism what is the balanced chemical equation for the overall reaction.
2HBrg O2gH2O2g Br2g Based on a kinetics study of the reaction represented by the equation above the following mechanism for the reaction is proposed. The particle models shown in the attached image represents a proposed two-step mechanism for the destruction of ozone O3 in the upper atmosphere. O fast CI The particle models shown above represent a proposed two-step mechanism for the destruction of ozone 03 in the upper atmosphere.
Kinetics table attached d Write a rate law for the reaction that is consistent with the mechanism. E A student claims that Cl2g is a catalyst in the reaction. 2O₃ g 3O₂ g Reasons.
The ozone molecule in the reaction consisting of three oxygen atoms is. Chlorine Cl2g to produce C2H9Clg and HClg. Mechanism 1Mechanism 2 X2 2 X slow X2 2 X slowX Y2 XY2 fast X Y2 XY Y fastX XY2 X2Y2 fastX XY X2Y fast X2Y Y X2Y2 fast Based on the information above which of the following is true.
Is believed to follow the following mechanism. The two step model for the destruction of ozone is presented as follows. Step 1HBrgO2gHO2BrgslowStep 2HO2BrgHBrg2HOBrgfastStep 32HOBrgH2O2gBr2gfast.
A proposed mechanism for the reaction which involves the free radicals HO and HOO is represented by the three equations below. 2A fast A B.

Writing Rate Laws Of Reaction Mechanisms Using The Rate Determining Step Chemical Kinetics Youtube

Pin On Festival Design Inspiration

Mammatus Clouds Over Bingley Uk Following A Thunderstorm On November 2 2013 The Last Proposed Formation Mechanism Mammatus Clouds Clouds Earth Atmosphere
0 Response to "Based on the Proposed Mechanism Which of the Following"
Post a Comment